What exactly is molar heat? It is the amount of energy required to raise the temperature of one mole of a substance by one degree Celsius.
Every substance has a specific molar heat, which is a measure of how easily it can be heated. For example, water has a high molar heat, which means that it takes a lot of energy to raise its temperature. In contrast, metals have a low molar heat, which means that they can be heated relatively easily.
The molar heat of a substance is important because it can be used to calculate the amount of energy required to heat or cool a given amount of that substance. This information is useful in a variety of applications, such as designing heating and cooling systems, and calculating the energy content of foods.
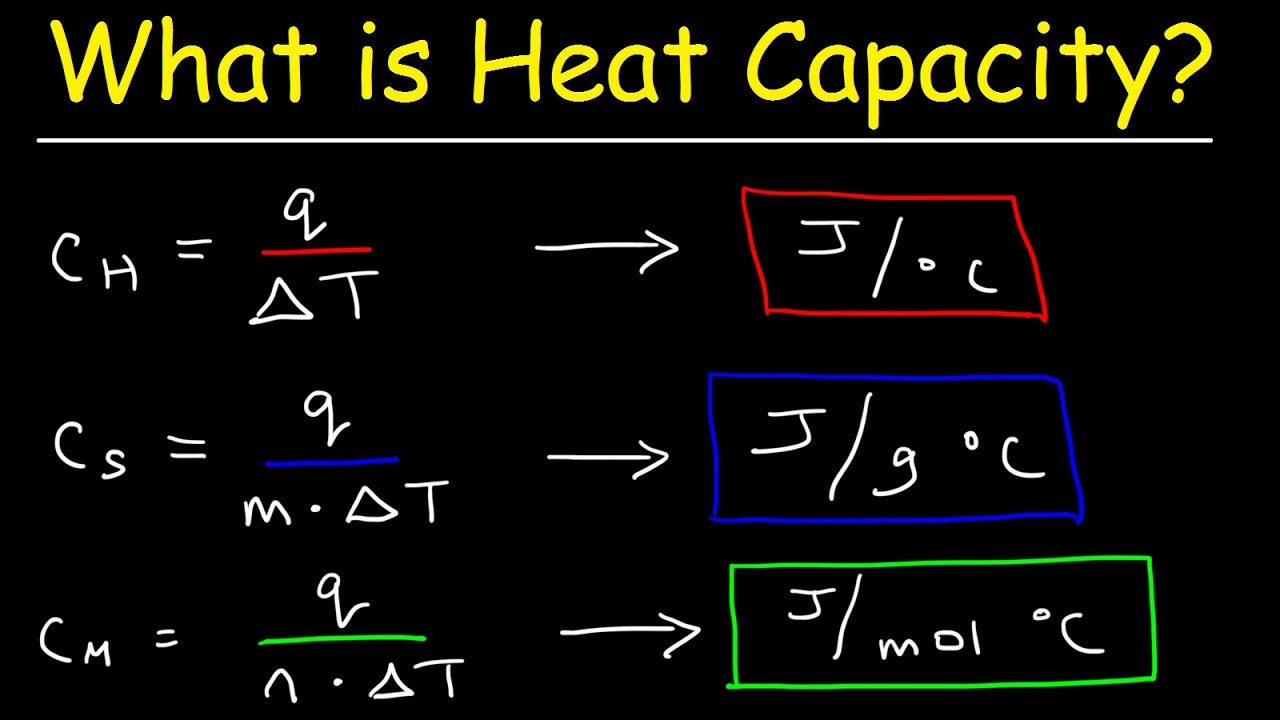
The molar heat of a substance can also be used to determine its specific heat capacity. The specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius. The molar heat and specific heat capacity are related by the following equation:
$$C_p = M c_p$$Where: Cp is the molar heat capacity in J/mol K M is the molar mass in g/mol cp is the specific heat capacity in J/g KMolar Heat
Molar heat is a crucial thermophysical property that quantifies the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius. It provides insights into a substance's thermal behavior and has wide-ranging applications in various fields.
- Magnitude: Molar heat values vary significantly across substances, indicating their relative ease or resistance to temperature changes.
- Composition Dependence: The molar heat of a compound is influenced by its molecular structure and composition, offering insights into intermolecular interactions.
- State Dependence: Molar heat can change with the physical state of a substance, reflecting differences in molecular arrangements and bonding.
- Temperature Dependence: For some substances, molar heat may vary with temperature, indicating changes in specific heat capacity or phase transitions.
- Applications: Molar heat finds applications in calorimetry, chemical engineering, material science, and other fields, enabling calculations of heat transfer, energy storage, and temperature control.
In conclusion, molar heat is a fundamental property that unveils the thermal characteristics of substances. Its variations and dependencies provide valuable information for understanding molecular behavior, designing thermal systems, and predicting energy requirements in various scientific and industrial contexts.
Magnitude
The magnitude of molar heat provides valuable insights into the thermal behavior of different substances. Substances with high molar heat values, such as water, require more energy to raise their temperature compared to those with low molar heat values, like metals. This variation is attributed to differences in molecular structure, intermolecular forces, and specific heat capacities.
For instance, water's high molar heat is due to the strong hydrogen bonding between its molecules, which requires significant energy to break. In contrast, metals have relatively weak metallic bonds, resulting in lower molar heat values and easier temperature changes.
Understanding the magnitude of molar heat is crucial for various applications. In engineering, it aids in designing thermal systems and predicting heat transfer rates. In chemistry, it helps determine the energy required for chemical reactions and phase transitions. Moreover, in materials science, it assists in selecting appropriate materials for specific thermal applications.
In summary, the magnitude of molar heat is a critical factor in understanding the thermal properties of substances. It reflects the relative ease or resistance to temperature changes and plays a vital role in numerous scientific and industrial applications.
Composition Dependence
The composition of a compound significantly influences its molar heat, providing insights into the nature and strength of intermolecular interactions. Compounds with similar molecular structures but different compositions exhibit varying molar heat values due to changes in intermolecular forces.
- Molecular Weight: Heavier molecules generally have higher molar heat values because more energy is required to increase their temperature. For example, butane (C4H10) has a higher molar heat than methane (CH4) due to its larger molecular weight.
- Functional Groups: The presence of polar functional groups, such as hydroxyl (-OH) or carbonyl (C=O), increases the molar heat of a compound. These groups enhance intermolecular interactions, such as hydrogen bonding or dipole-dipole interactions, requiring more energy to overcome during temperature changes.
- Molecular Geometry: Compounds with complex molecular geometries often have higher molar heat values compared to those with simpler geometries. Complex geometries hinder efficient molecular packing, leading to weaker intermolecular forces and higher molar heat.
- Crystalline Structure: The arrangement of molecules in a crystal lattice influences molar heat. Crystalline compounds with ordered structures typically have lower molar heat values than amorphous compounds with disordered structures. This is because the ordered arrangement in crystals promotes stronger intermolecular interactions.
Understanding the composition dependence of molar heat is crucial for predicting the thermal behavior of compounds. It aids in the design of materials with desired thermal properties, such as thermal insulators or heat transfer fluids. Moreover, it provides insights into the intermolecular interactions that govern the physical and chemical properties of substances.
State Dependence
The physical state of a substance significantly influences its molar heat, providing insights into the changes in molecular arrangements and bonding that occur during phase transitions.
- Solid to Liquid: When a solid melts, its molar heat increases. This is because the molecules gain energy and overcome the strong intermolecular forces holding them in a fixed lattice structure. As a result, they become more mobile and require more energy to raise their temperature.
- Liquid to Gas: The molar heat of a substance further increases when it transitions from a liquid to a gas. This is due to the significant energy required to overcome the intermolecular forces and break the molecules apart into individual, non-interacting entities.
- Gas to Plasma: In the case of certain substances, the molar heat continues to increase upon ionization and transition to a plasma state. This is because plasma consists of free ions and electrons, which require even more energy to raise their temperature.
- Amorphous vs. Crystalline: For some substances, the molar heat can differ depending on whether they are in an amorphous or crystalline state. Crystalline solids have a more ordered molecular arrangement, leading to stronger intermolecular interactions and lower molar heat compared to amorphous solids with disordered structures.
Understanding the state dependence of molar heat is crucial for predicting the thermal behavior of substances and designing processes involving phase transitions. It provides valuable insights into the energetics of these transitions and helps optimize industrial processes, such as melting, casting, and vaporization.
Temperature Dependence
The temperature dependence of molar heat provides valuable insights into the thermal behavior of substances, revealing changes in their specific heat capacities and phase transitions.
- Specific Heat Capacity: For some substances, molar heat increases linearly with temperature. This indicates an increase in specific heat capacity, which is the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius. This behavior is often observed in solids and liquids as their temperature increases.
- Phase Transitions: Molar heat can also exhibit abrupt changes at specific temperatures, corresponding to phase transitions. For example, the molar heat of water shows a sharp increase at its melting point and boiling point, indicating the energy required to overcome intermolecular forces and transition between solid, liquid, and gas phases.
- Molecular Vibrations: In gases, molar heat may increase with temperature due to an increase in the amplitude and frequency of molecular vibrations. This is because higher temperatures provide more energy for molecules to vibrate, requiring more energy to raise their temperature.
- Intermolecular Interactions: In liquids, molar heat can change with temperature due to changes in intermolecular interactions. As temperature increases, intermolecular forces may weaken, leading to a decrease in molar heat. This behavior is observed in some organic liquids and molten salts.
Understanding the temperature dependence of molar heat is crucial for accurate predictions of heat transfer and temperature control in various applications. It helps optimize processes involving heating, cooling, and phase transitions, ensuring efficient energy usage and desired material properties.
Applications
Molar heat plays a crucial role in various scientific and industrial applications, providing essential insights into the thermal behavior of substances. Its practical significance lies in enabling precise calculations related to heat transfer, energy storage, and temperature control.
In calorimetry, molar heat is used to determine the heat capacity of substances, which is a measure of their ability to absorb and release heat. This information is vital for designing and optimizing heating and cooling systems, as well as predicting the thermal behavior of materials in different environments.
In chemical engineering, molar heat is used to calculate the energy requirements for chemical reactions. This knowledge is essential for process design, optimization, and safety assessments. It helps engineers determine the appropriate operating conditions, reactor sizes, and cooling systems to ensure efficient and safe chemical processes.
In material science, molar heat is used to study the thermal properties of materials and design materials with specific thermal characteristics. For instance, understanding the molar heat of different polymers allows scientists to develop materials with tailored thermal conductivities for applications such as insulation, heat dissipation, and thermal energy storage.
In conclusion, molar heat is a fundamental property that underpins various applications across multiple disciplines. Its practical significance lies in enabling accurate calculations related to heat transfer, energy storage, and temperature control. By leveraging our understanding of molar heat, scientists and engineers can design and optimize systems for efficient energy utilization, thermal management, and material performance.
Frequently Asked Questions (FAQs) about Molar Heat
This section aims to address common questions and misconceptions surrounding molar heat, providing concise and informative answers to enhance understanding.
Question 1: What is molar heat, and how is it measured?
Molar heat, denoted by the symbol Cp, is the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius. It is typically measured in units of joules per mole Kelvin (J/mol K).
Question 2: Why does molar heat vary among different substances?
The molar heat of a substance depends on its molecular structure, composition, and intermolecular interactions. Substances with stronger intermolecular forces and more complex molecular structures generally have higher molar heat values.
Question 3: How is molar heat related to specific heat capacity?
Molar heat and specific heat capacity are related through the following equation: Cp = M * c, where Cp is the molar heat, M is the molar mass, and c is the specific heat capacity. Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius.
Question 4: What are some practical applications of molar heat?
Molar heat finds applications in various fields, including calorimetry, chemical engineering, and material science. It is used to calculate heat transfer rates, determine the energy requirements for chemical reactions, and design materials with specific thermal properties.
Question 5: Can molar heat change with temperature or pressure?
In some cases, molar heat may vary with temperature or pressure. For gases, molar heat typically increases with temperature due to increased molecular vibrations. For liquids and solids, molar heat may change with pressure due to changes in intermolecular interactions.
Question 6: How is molar heat determined experimentally?
Molar heat can be determined experimentally using various methods, such as calorimetry and differential scanning calorimetry (DSC). These techniques involve measuring the temperature change of a known mass of a substance when a known amount of heat is applied.
In summary, molar heat is a crucial thermophysical property that provides insights into the thermal behavior of substances. Understanding molar heat enables scientists and engineers to design and optimize systems for efficient energy utilization, thermal management, and material performance.
Transition to the next article section:
This concludes the FAQ section on molar heat. For further exploration, the next section will delve into advanced concepts and applications of molar heat in various scientific and industrial fields.
Conclusion
In this article, we have explored the concept of molar heat, its significance, and its diverse applications across scientific and industrial domains. Molar heat provides crucial insights into the thermal behavior of substances, enabling researchers and engineers to design and optimize systems for efficient energy utilization, thermal management, and material performance.
Understanding molar heat is essential for advancements in various fields, including calorimetry, chemical engineering, and material science. By leveraging our knowledge of molar heat, we can develop innovative materials with tailored thermal properties, optimize chemical processes for energy efficiency, and design systems for precise temperature control. The continued exploration of molar heat and its applications holds promising prospects for scientific discoveries and technological breakthroughs.
Most Comprehensive Guide To Azure Subscription Types
Kohl's: Who's The Owner Behind The Retail Giant?
The Key Features Of Cloud Computing: What You Need To Know

Molar Specific HeatSpecific heat, And Types

Molar Heat Capacity Problems Physics YouTube
What Is The Difference Between Specific Heat Capacity, Heat Capacity